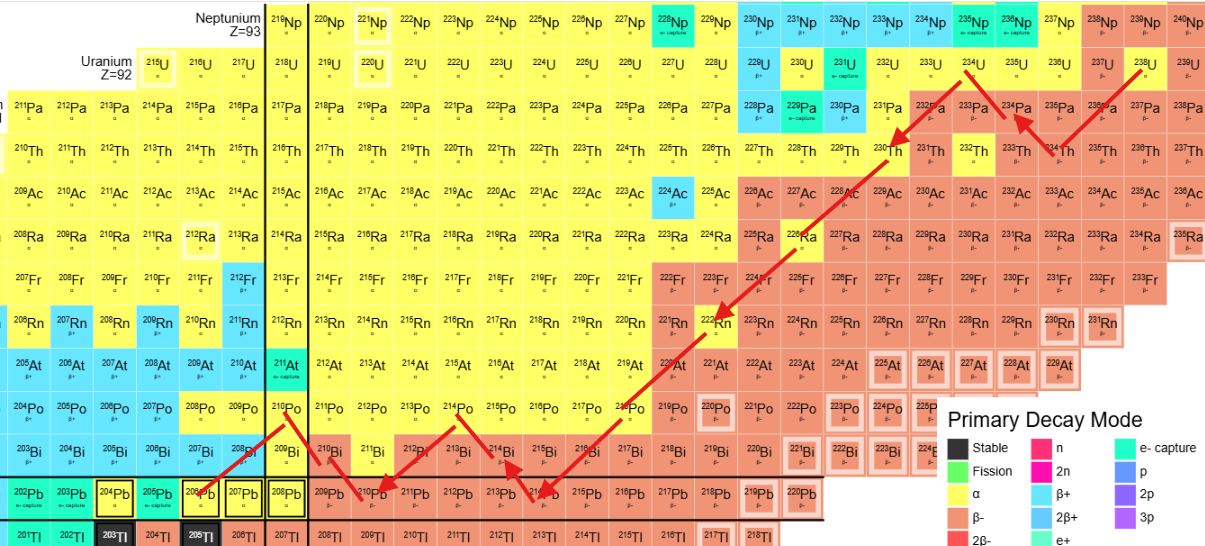
This diagram shows the decay series for Uranium-238 ending in stable Lead-206 created by following the primary decay mode for each nuclide. There are other paths but typically these have very much lower probability. (ignore the arrow-heads)

The cell colour shows the primary decay mode: Yellow for Alpha (emission of a helium nucleus, two protons and two neutons), Brown for Beta- (change of neutron to a proton and an electron). Each decay moves the atom through the chart: Alpha: two steps left (2 less neutrons), two steps down (2 less protons), Beta- : 1 step diagonally left and up (1 less neutron, 1 more proton + electron).
Follow through the chart from U-235 and then from Th-232. Hopefully the decay ends in the predicted Lead isotopes.